In the last issue, I walked through the presentations to the panel of independent experts at the CDC’s ACIP meeting in favor of the new bivalent vaccine booster. These presentations focused on the low levels of efficacy we have been seeing from both the vaccine and the subsequent boosters and heavily imply that the newest booster is a necessity for protection against COVID coming into the winter season.
It’s important to understand the role that the ACIP recommendations play in vaccine administration. ACIP is comprised of 15 voting members selected to represent the breadth of research and practical medicine concerning vaccine administration. Voting ACIP members may not participate directly in vaccine research, as that would be a conflict of interest. They also may not be employed by or have any direct financial interest in vaccine development or manufacture. ACIP aims to draw from the highest levels of expertise while forbidding even a hint of economic interest.
This is, I think, the best possible approach. It is safeguarded against a number of potential pitfalls and explicitly designed to protect the interests of the public. Which only makes what happened last month that much more grotesque.
To understand what happened in last month’s ACIP meeting, we have to keep our eye on the recommendation placed before them for a vote. I’m honestly not sure where this recommendation came from. Perhaps it was developed by the FDA to align with the emergency use authorization or developed by the CDC to match the FDA’s work. However it came about, it was certainly not crafted by the ACIP members. The recommendation that they ended up approving reads as follows:
A single booster dose of the bivalent Pfizer / Moderna COVID-19 vaccine is recommended for individuals ages 12 years and older for Pfizer (18 years and older for Moderna) and at least 2 months after receipt of a primary series or prior monovalent booster dose, under the EUA issued by the FDA.
How this recommendation was approved is a fascinating journey of objection and frustration among some of the least confrontational, most helpful doctors in the country.
To set the stage for this discussion, we have to understand that, even before this meeting, the FDA had revoked the Emergency Use Authorization (EUA) for the previous vaccine booster in anticipation of the approval of the bivalent vaccine booster. This means that if the bivalent booster recommendation was not approved, no COVID booster would be available to anyone, not even the most vulnerable or aged citizens. The choices were either to recommend this booster or to have no booster available. There was nothing in between.
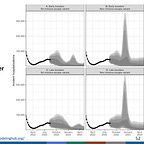
Compound that reality with this chart (presentation source) presented to the ACIP members. The model that generated this chart was not called into question, and most ACIP members simply assumed that the failure to approve this booster vaccine would, in fact, result in nearly 10,000 deaths.
With all that background, let’s talk about how the ACIP bivalent recommendation went down.
After the presentations, there was a time for debate and comment from the ACIP members. You can watch that on YouTube and, if you’re not interested in listening to all 3 hours of it, you can use my notes from the presentation as a guide to find the most important parts.
There were a few big issues that the ACIP members had with this booster. The biggest problem was voiced by Dr. Pablo Sanchez, who noted that the booster vaccine that they were supposed to be recommending had no clinical data associated with it, which is to say it had not yet been tested on humans. This booster is targeting the BA.4/BA.5 variant but the clinical data was about a version of the booster that targeted the BA.1 (which is still considered Omicron).
The vaccine boosters approved in Europe were for the BA.1 vaccine, but the US government had already purchased 171 million doses of this newer but untested vaccine. This put enormous pressure on the ACIP panel. They couldn’t recommend the BA.1 vaccine partly because there wasn’t an FDA’s EUA for it, but also because it simply wasn’t manufactured for use in the US. Securing enough doses of the tested vaccine would take months.
The fact that the FDA had already issued an EUA for this vaccine came up as a problem again and again. There were complaints about the recommended age range for the booster. Participants suggested that the CDC recommendation be altered to recommend this only for people over 50. They were told that the wording around age recommendations could not be changed without causing substantial legal problems related to the EUA.
The committee also argued against the “at least 2 months” recommendation, saying that the booster should be administered at least 3 months and ideally 6 months after a COVID infection. Again, they were told that the wording could not be changed without threatening the EUA and thus leaving elderly and vulnerable people without any booster for the coming winter months.
It’s incredible to take a step back and look at what has happened here. The CDC came to their independent experts with an untested vaccine, presented them with a recommendation to which they objected on multiple grounds, and informed them that, if they did not approve the given recommendation, there would be no booster for the elderly and vulnerable and 10,000 people would die.
The only thing that placated the experts was the assurance that their true recommendations would be included in the clinical considerations. In this assurance, the CDC simply lied to its experts. None of this information has been presented in the clinical considerations document.
As an added insult, Ashish Jha, the White House COVID Response Coordinator, has been working with “employers, colleges, and schools” to roll out the new boosters. That is to say, they are specifically targeting younger people, the group that the ACIP participants specifically said should not be targeted.
I’m disappointed that the ACIP did end up voting for the recommendation, but I’m astonished at the disdain with which the CDC treated its expert panel. They presented these doctors with an all-or-nothing recommendation and ignored every objection that was brought up. By revoking the old booster EUA, they boxed these experts into a corner and told them that they would be responsible for thousands of deaths if they refused to approve.
And they didn’t seem even the slightest bit embarrassed by this. If anything, they seemed incredulous that there was any objection at all. At one point, the committee was told that complaints about authorizing an untested vaccine should not be made because “we cannot go backward and change things [ie manufacture the BA.1 booster instead of the BA.4/BA.5 booster], so I’m going to suggest that any additional comments (should) look ahead, look forward. We can’t change what is put in front of us today.”
This disdain for the advice of independent experts in favor of rubber-stamping a historically controversial vaccine was incredibly distressing and antithetical to the intended purpose of the ACIP group. My main comfort in all this is at least it is happening out in the open. At least everyone can see the presentations and listen to the objections the experts had to the CDC’s awful recommendation.
Disney Shorts: The Nifty Nineties (1941)
This short presents Mickey and Minnie as denizens of the 1890’s, which must have been really fun for audiences in the 40’s to see the recent past nostalgically portrayed this way. I’ve long contended that the point of Back to the Future wasn’t “let’s see our parents as kids” but a wide-eyed step back to marvel at how much had changed in 30 short years. In the same way, The Nifty Nineties shows Mickey and Minnie in a Brass Era car, attending a vaudeville show, and watching a morality tale slideshow against the dangers of alcohol.
The short also featured music authentic to the 1890’s. It’s fairly well in step with Disney’s tender romanticization of small town life and romanticization of the recent past.